Biomechanics Data
VYRSA™ V1
An Evaluation of Integrated Lateral Transfixation Technology
A New Mechanism of SI Joint Stabilization:
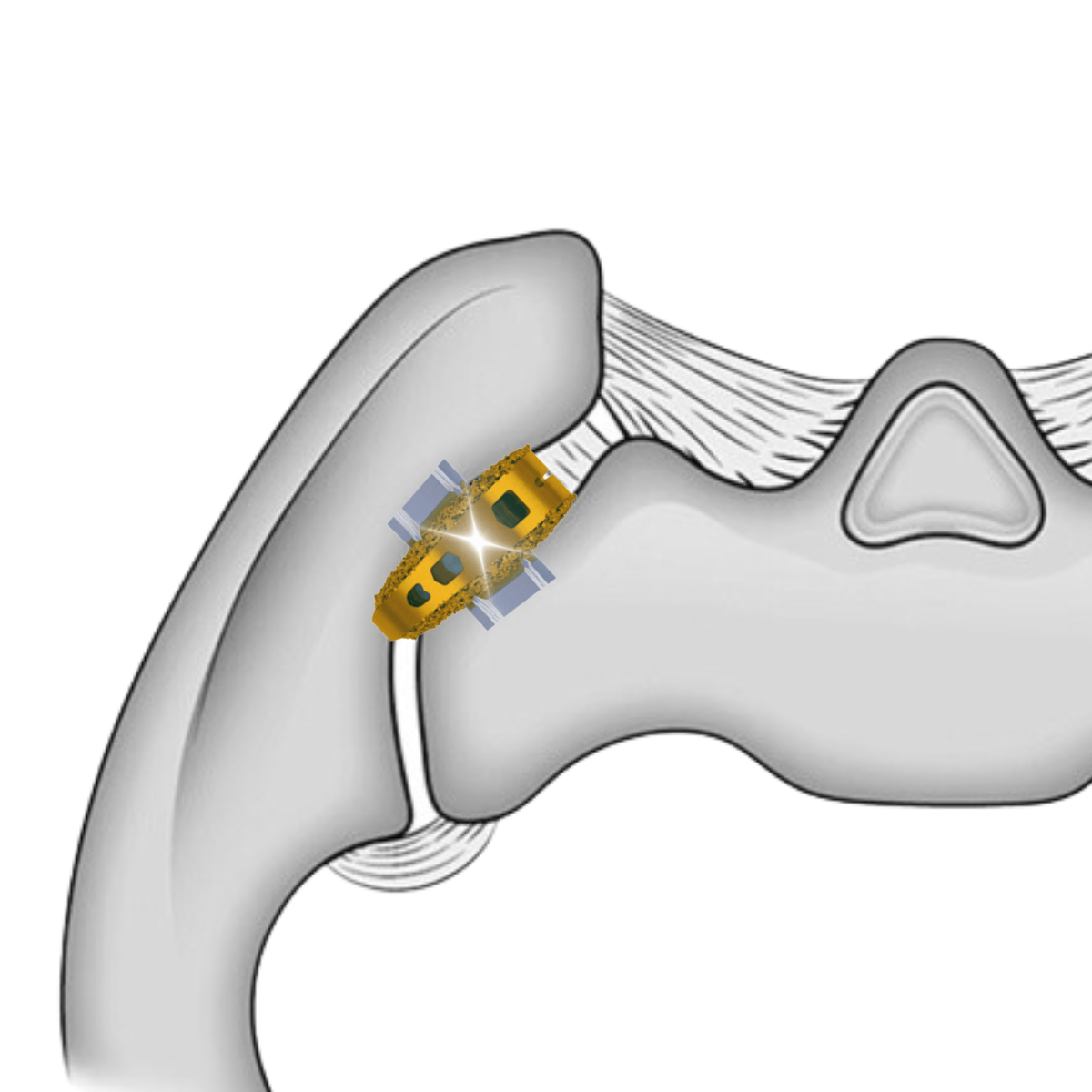
The VYRSA™ V1 implant is initially delivered into the sacroiliac joint through a posterior approach. Once in place, titanium anchors deploy laterally into the sacrum and illium providing axial and rotational stability of the joint.
A Comprehensive Biomechanics Analysis
In Q4 of 2022, the Camber SI Implant received an FDA 510k clearance for sacroiliac joint fusion. This came after a rigorous 2+ year biomechanics analysis project performed in collaboration with the FDA to establish effective stabilization of the SI joint.
Project Timeline:
Study Title:
Preclinical Characterizations of the Camber SI and Siconus Fixation Systems
Testing Facility:
Spine Research Lab, Allegheny Health Network, Allegheny Singer Research Institute, Department of Neuroscience
Principal Investigator:
Boyle Cheng, PhD
Professor of Neurosurgery, Drexel University College of Medicine
Director of Neurosurgical & Spine Research, Allegheny General Hospital
100+ publications on spine biomechanics
Introduction:
“Non-autoimmune sacroiliac joint (SIJ) dysfunction is reported to be responsible for 15 – 30% of chronic low back pain [1, 2]. Minimally invasive sacroiliac joint fusion has become an increasingly popular approach to the remediation of this chronic pain. While there is no universally accepted gold standard for diagnosing or surgically treating SIJ pain, these new fusion procedures have been associated with improved pain relief, disability, and quality of life with relatively high satisfaction rates [3].Recently there has been an increased interest in the posterior or dorsal approach to the sacroiliac joint, which involves the placement of one or two cortical allografts or interbody devices along the joint[4-6]. This new approach has yet to be studied to the extent from a clinical or biomechanical perspective and poses new challenges due to the irregular anatomy of the SIJ.”
Background:
“According to a survey conducted amongst spinal surgeons, the prevalence of minimally invasive SIJ fusion procedures has more than doubled from 2009 to 2012 from 39% to 87% of SIJ fusions being performed with a minimally invasive approach [7]. Directly related to the increase in frequency seen with this procedure has been an increase in interest from medical device companies in the field. The mechanism of pain relief with these implants is thought to be two-fold with early pain relief as a result of immediate surgical joint stabilization and late pain relief as a result of surgical stabilization and long-term fusion [8]. While initial reports have indicated positive results regarding minimally invasive SIJ fusion, the rapidly growing interest from both surgeons and medical device companies indicates that research into the procedure is warranted in order to understand more about its efficacy and mechanism.”
Study Design:
“This report is intended to assess the biomechanical stability of the sacroiliac joint (SIJ) in cadaveric lumbopelvic specimen instrumented with either the Camber SI Fixation System (Test) or SICONUS (Control) instrumentation system.”
FDA Concerns Addressed:
-
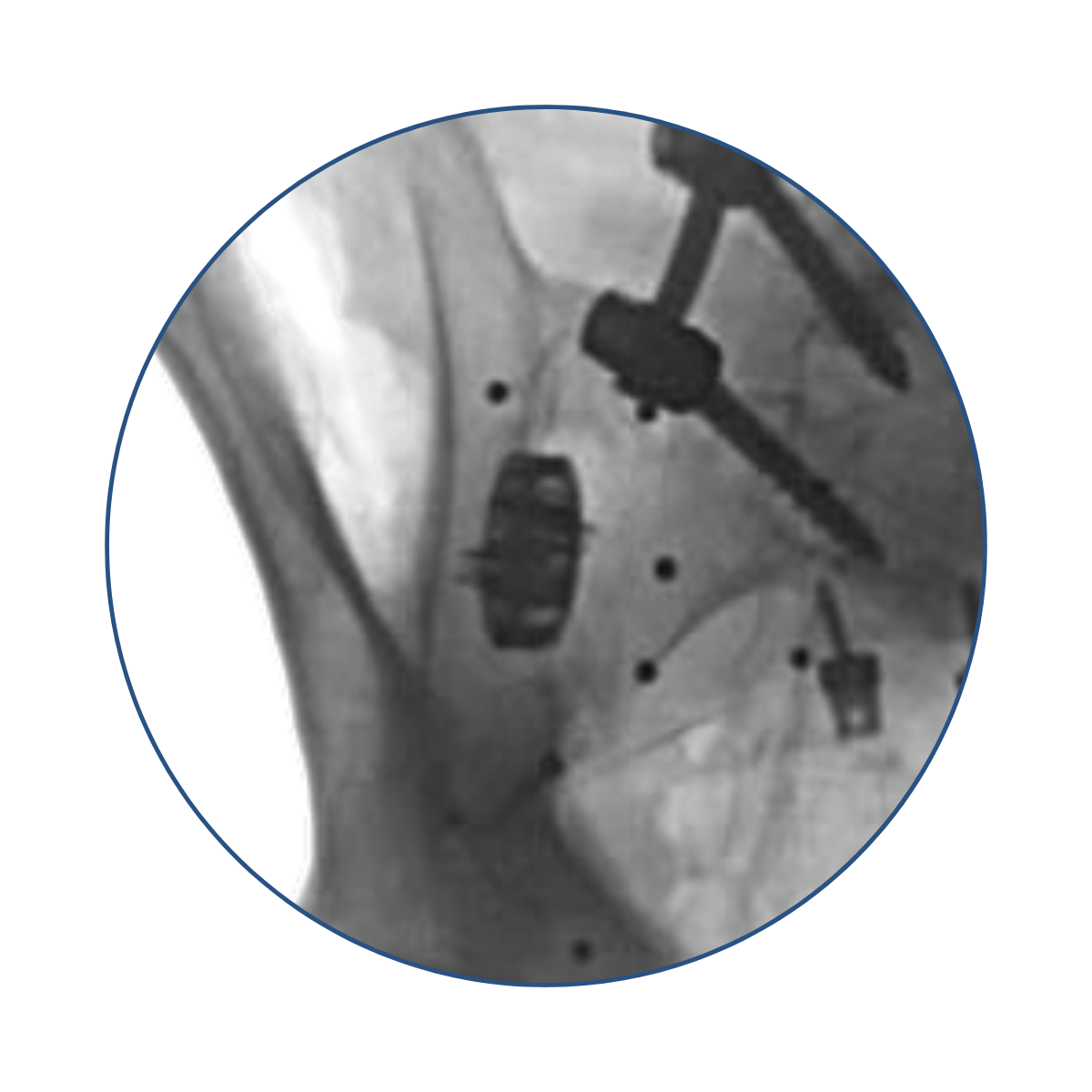
Cadaveric labs were meticulously performed in collaboration with expert spine surgeons to validate the safe and reliable placement of the V1 implant within the sacroiliac joint space.
-
Cadaveric lab studies were performed to evaluate the risk of neuromuscular impingement during implantation. The results showed that the V1 implant reduced the probability of this complication in comparison to the lateral screw predicate devices.
-
Detailed analysis of sacroiliac joint implanted with the V1 implant showed a statistically insignificant increase of joint distraction.
“Unintended distraction was quantified at three points along the anterior aspect of each implanted… On average, the distractions measurements were all less than 1 mm regardless of the location along the SIJ where measurements were taken, indicating a low risk of unintended distraction.”
*Data on File
-
24 rounds of biomechanics testing were performed analyzing 30 different measurements of SI joint motion after implantation with the VYRSA™ V1 implant.
Summary:
30
Performance Measurements
100k
Cyclical Loads Analyzed
24
Rounds of Cadaveric Trials
2
Year Testing Process
30 performance measurements were analyzed to determine the level of stabilization provided by the be VYRSA™ V1 and control group. These measurements included:
Measurements:
Flexion Stiffness
Extension Stiffness
Lateral Bending Stiffness Toward the Fixed Ischium
Lateral Bending Stiffness Away from the Fixed Ischium
Axial Torsion Stiffness Toward the Fixed Ischium
Axial Torsion Stiffness Away from the Fixed Ischium
Axial Torsion Stiffness Toward the Fixed Ischium
Joint Distraction
Flexion Extension
Lateral Bending
Axial Torsion
Axial Compression
Superior Inferior Translation
Medial-Lateral Translation
Anterior-Posterior Translation
Neutral Zone
Study Results:
Vs.
"We found no significant differences between the Test and Control implant groups with respect to any of the outcome measures investigated. Across all biomechanical measures and analyses we found no statistically significant differences between the Camber SI Fixation System and SICONUS implant groups."