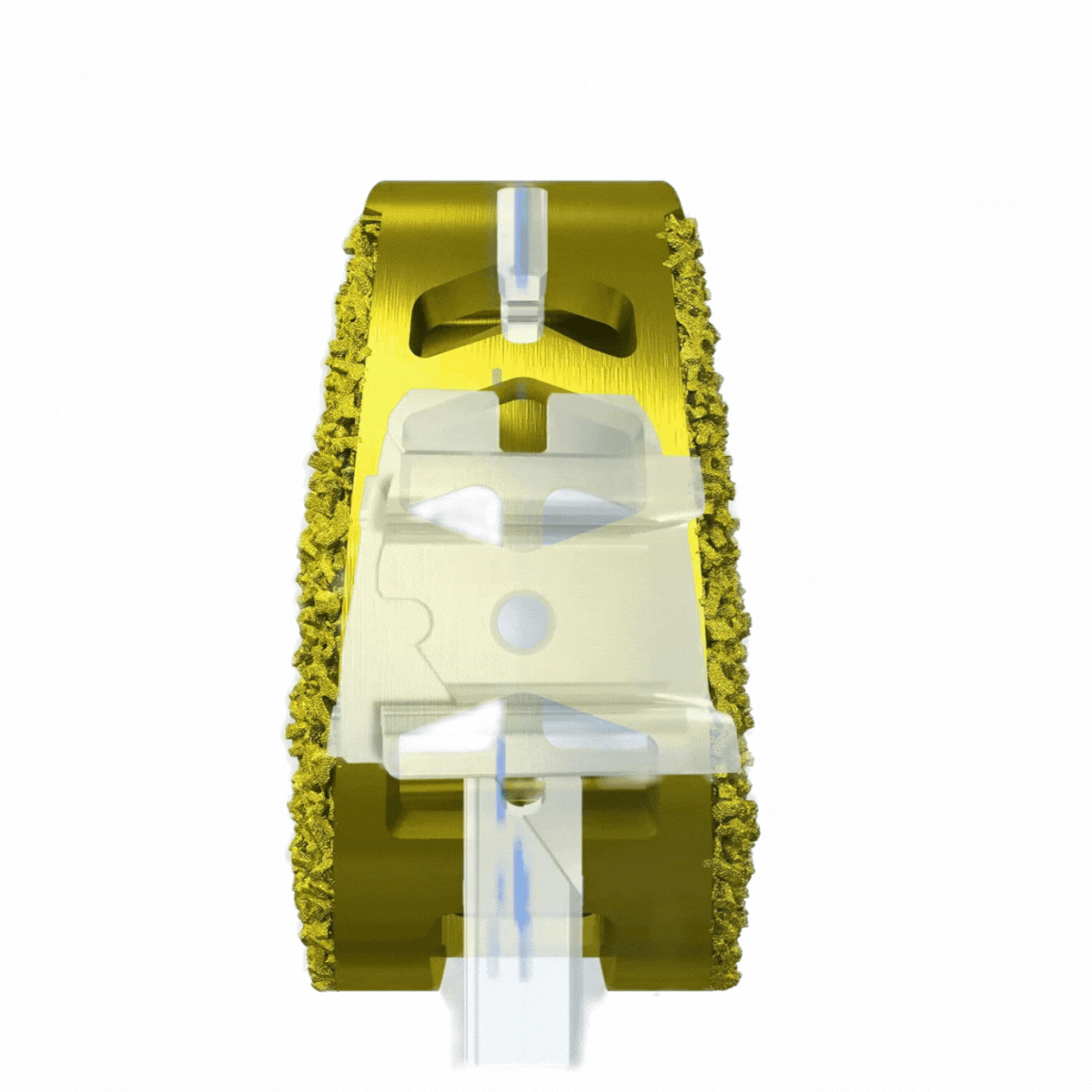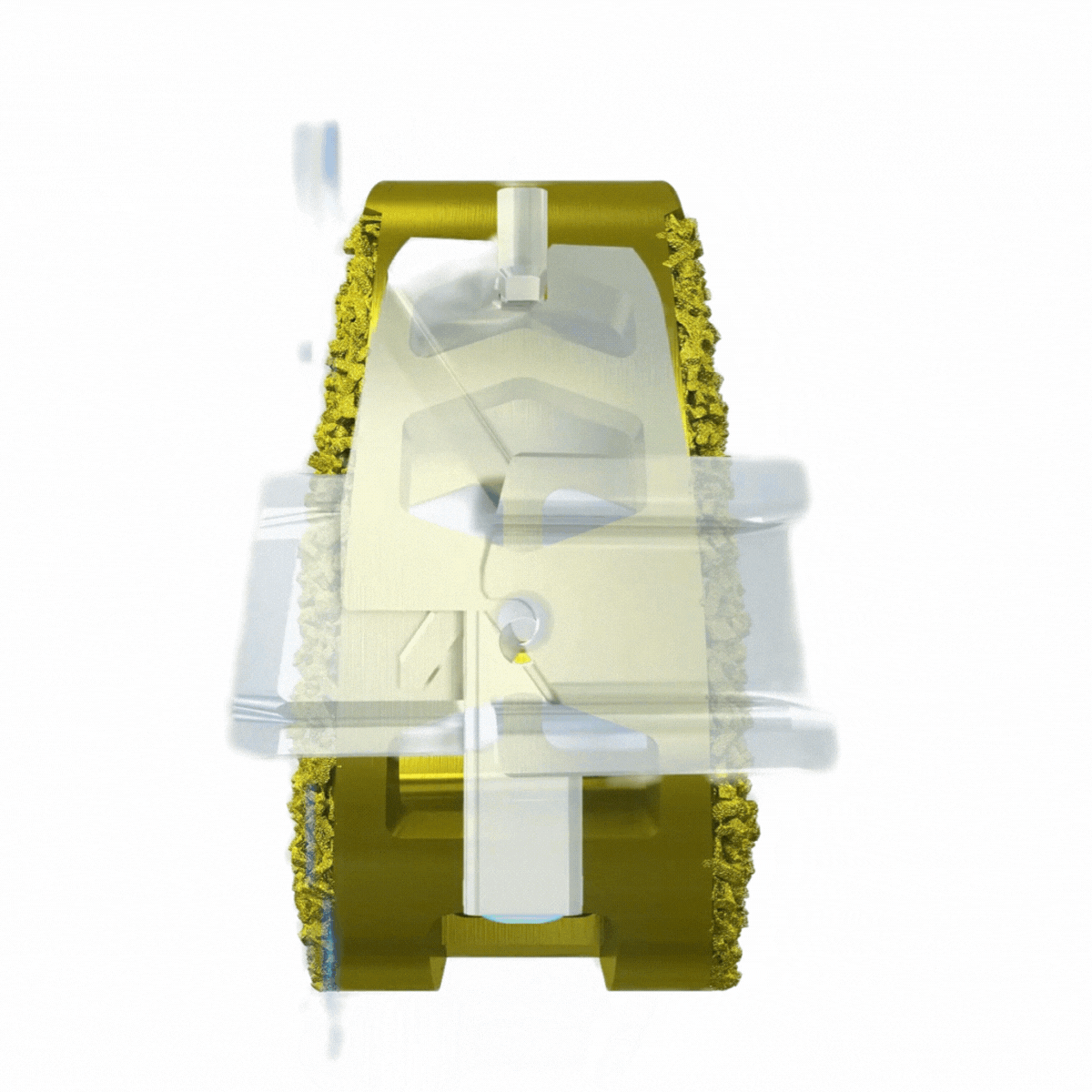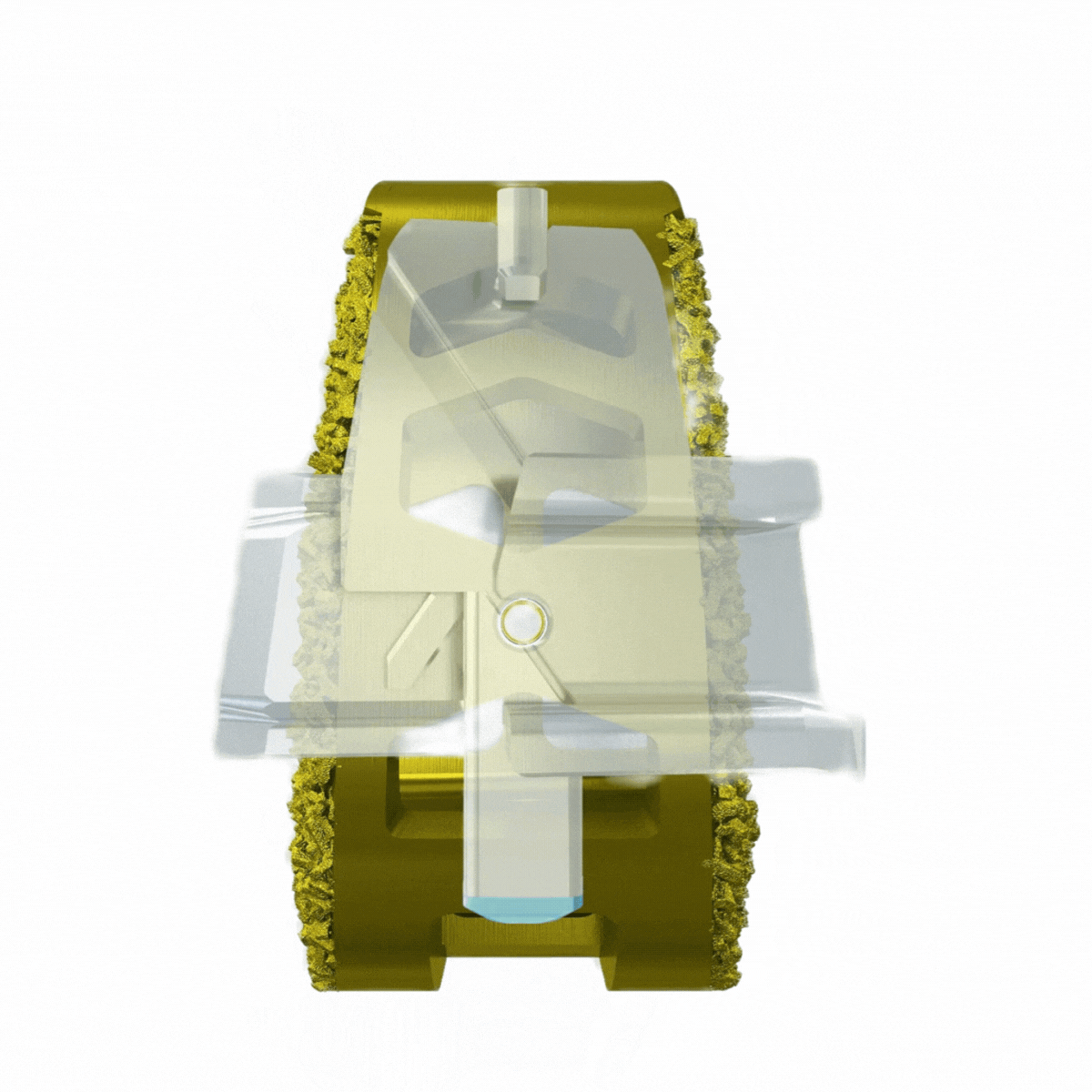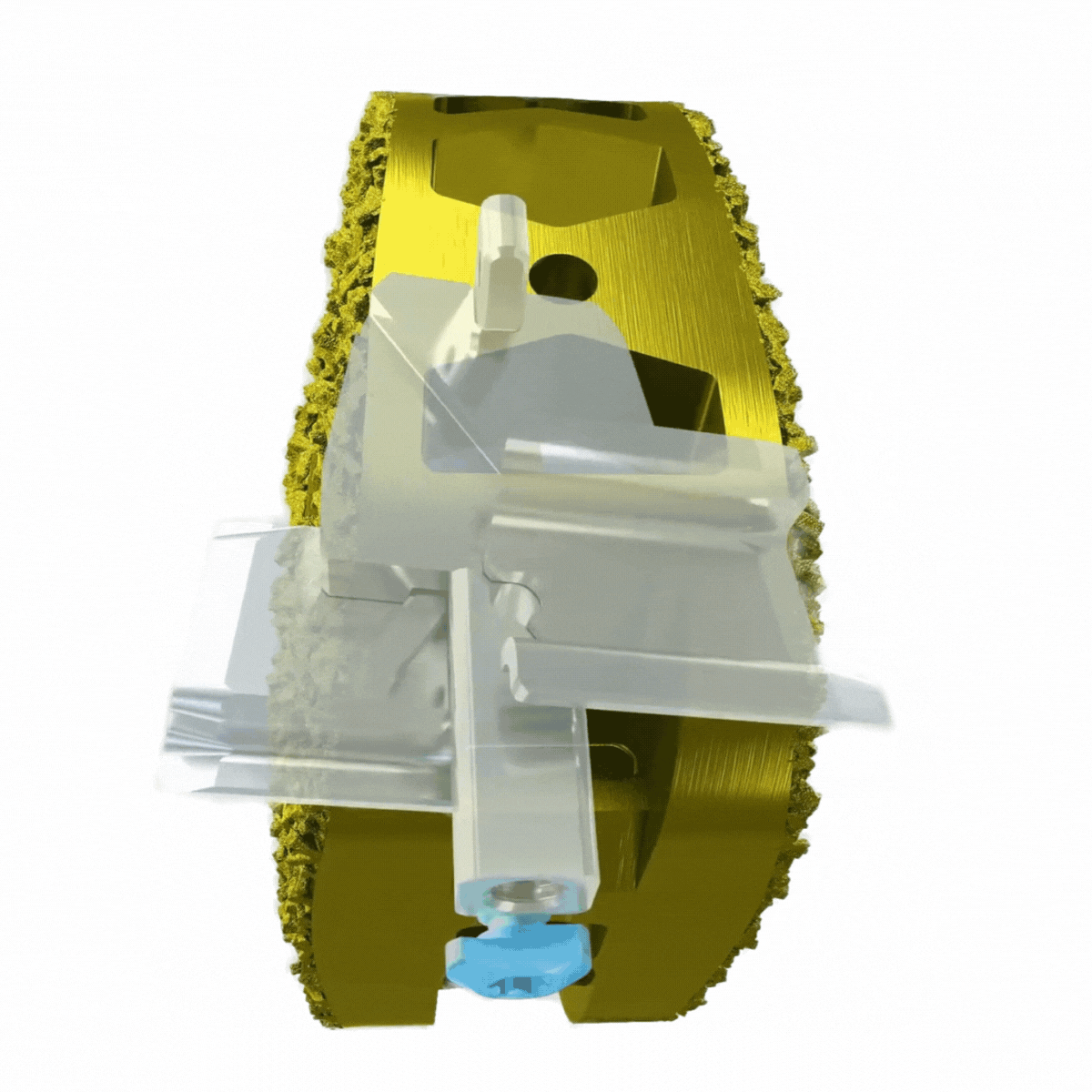
The Future of Sacroiliac Joint Fusion Has Arrived.
The Nevro1™ SI Joint Fusion System is now FDA-cleared for sacroiliac joint fusion.
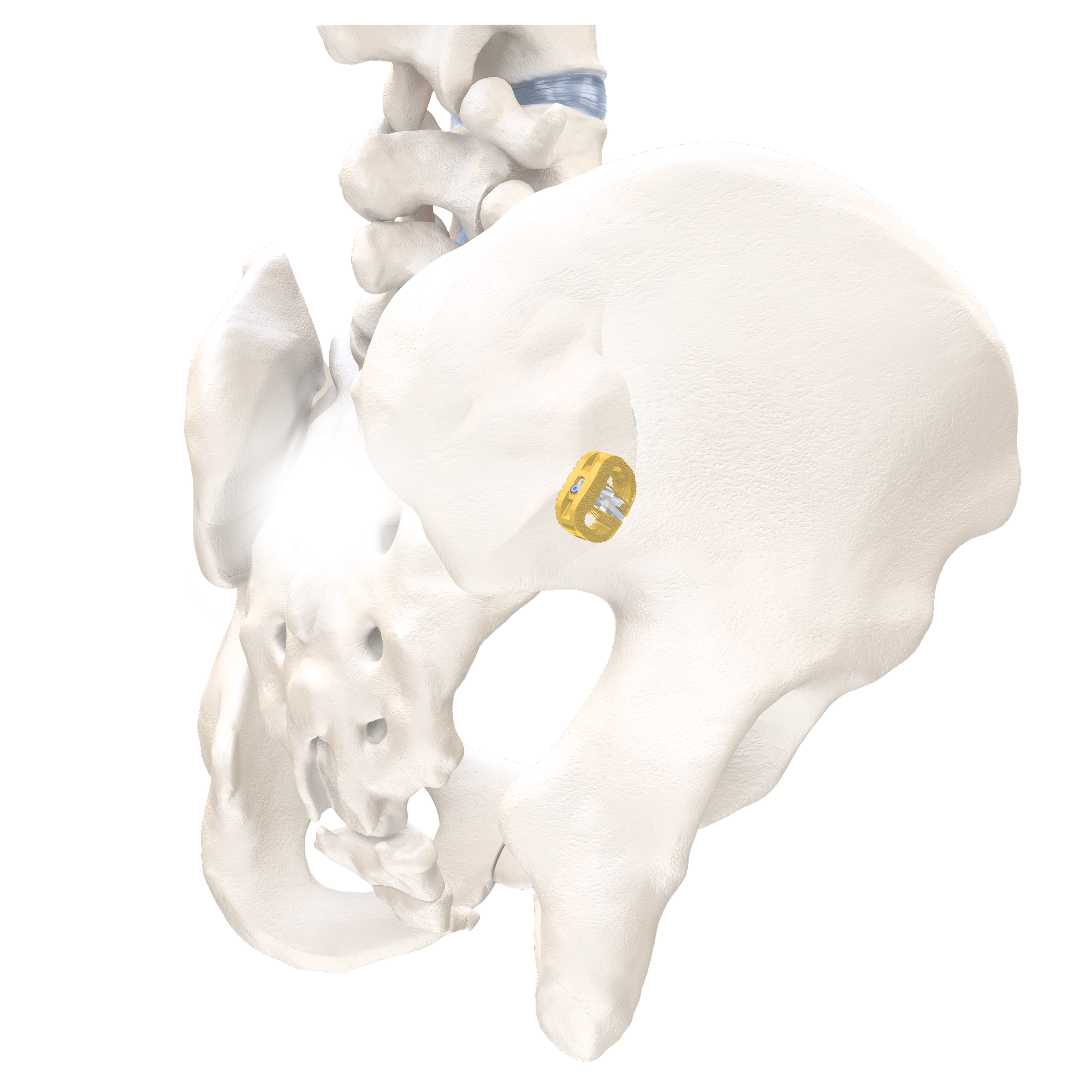
Immediate Sacroiliac Joint Stabilization.
Self-contained, deployable titanium anchors transfix the sacrum and ilium to enhance axial and rotational stability of the joint.
Endplate penetration promotes bone bleeding into graft.
Immediate stabilization may lead to lower post-op pain scores.
*DATA ON FILE

Backed by Clinical Data.
An exhaustive 2.5-year study of SI Joint stabilization with the Nevro1 implant in comparison to 2 lateral screws produced zero statistically significant differences in performance across 30 individual measurements of stability.
*DATA ON FILE
3-D Printed Bone-Growth Enhancing Technology.
An osteopromotive surface engineered for promotion of bone cell differentiation and proliferation with subsidence resistant geometry.
Pores averaging 500µm in diameter – the optimal environment for arthrodesis to occur.
Deliberately designed to achieve bony-implant through-growth.
*DATA ON FILE
-
The Nevro1™ Sacroiliac Transfixing and Fusion System is intended for sacroiliac joint fusion for conditions including sacroiliac joint disruptions and degenerative sacroiliitis.
CAUTION: FEDERAL LAW (USA) RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN.
The Nevro1™ Effectively Transfixes the Sacroiliac Joint.
The V1™ Implant is an FDA 510k-cleared device intended to transfix the sacroiliac (SI) joint for immediate stability and long term fusion. The mechanism of stabilization consists of titanium anchors deployed laterally through the ilium, across the sacroiliac joint and into the sacrum. This device has been proven to substantially reduce SI joint motion through comprehensive biomechanics testing performed in collaboration with the FDA*.
*CPT Category III codes long descriptors – updated November 1st, 2022 - page 348, Table 46, : FINAL CY 2023 SI AND APC FOR CPT CODES 27279 AND 0775T - Medicare Program: Hospital Outpatient Prospective Payment and Ambulatory Surgical Center Payment Systems and Quality Reporting Programs. Click here to view document.
*Preclinical Characterization of Camber SI and SICONUS Fixation Systems, B. Cheng, 2022
Project Timeline: